Supervisors: Prof. Alon Gany, Dr. Valery Rosenband
Ph.D Student: Ms. Shani Elitzur
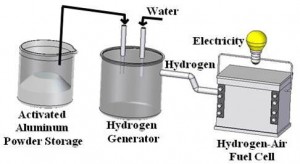
A novel method of hydrogen production via a self-sustained reaction between powdered aluminum and water has been proposed, investigated, and patented. Typically, aluminum is almost not reactive with water because of the existence of an oxide or hydroxide film on the particle surface, preventing fast chemical interactions, with the surroundings. The novelty in this invention is an original thermal-chemical treatment of the particles, causing activation of the aluminum powder. The method developed in-house is simple and efficient, indicating a breakthrough in hydrogen technology. The aluminum treatment by a lithium-based activator causes modification of the surface film , to be non-protective and substantially improving the interaction between the metal and surrounding water according to the following reaction:
Al + 3H2O —> Al(OH)3 + 3/2 H2
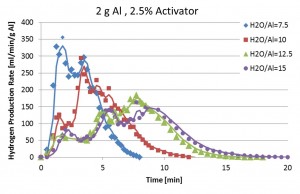
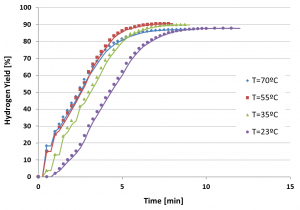
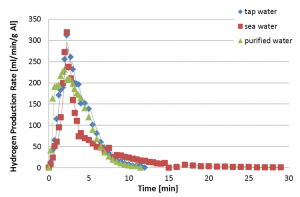
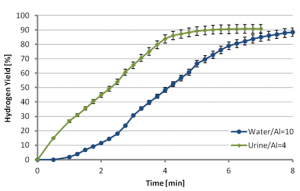
Any type of water (tap water, sea water or waste water) can be used for the aluminum-water reaction. Experiments conducted have revealed that a fast, self sustained reaction of the activated aluminum with water can take place at room temperature, yielding reaction efficiency of 90% and more, generating about 1.2 liter of hydrogen per gram of aluminum. The reaction rate may be controlled by the aluminum particle size, water temperature, metal activation conditions, and metal/water mass ratio.
Advantages:
- Very safe and compact hydrogen storage: 11% of hydrogen mass compared to the aluminum mass, yielding overall hydrogen density substantially higher than that of hydrogen gas in pressurized vessels, or liquid hydrogen.
- On-site and on-demand production and controlled supply of hydrogen, using any form of available water.
- Energy “bonus”: the aluminum-water reaction releases 17 kJ per gram of aluminum.
- Non-polluting: the solid state residues (aluminum oxide or hydroxide), can be further used (particularly as a fire retardant) or recycled back to aluminum (usually via electrolysis).
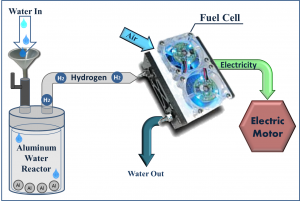
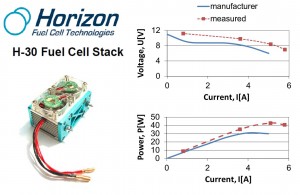
From Aluminum to Electricity via Fuel Cell
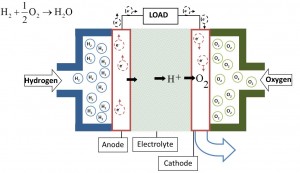
The hydrogen generated in this aluminum-water system may be applied for electric energy generation via fuel cells. According to the chemical reaction H2 + 0.5O2 →H2O + electric energy, it is especially attractive for mobile and stand-alone applications on earth, air, sea, and space, including direct automotive and marine propulsion,unmanned vehicles, backup systems, and emergency generators. It presents very high specific electric energy storage (up to 2200 Wh/kg Al and about 700-2000 Wh per kg of the power system, depending on the mission type and duration, compared to 120-150 Wh/kg for commonly used lithium-ion batteries and 200-250 Wh/kg for special high performance chargeable batteries).
 |
 |